
Molecular docking study was carried out to predict the potency of inhibition of L against mitochondrial Ubiquinol-Cytochrome C Reductase binding (UQCRB) protein. + (SC 50 8.55 ± 0.17 μg/mL) scavenging activities compared with standard antioxidant compounds such as Trolox (TRO), rutin (RUT) and butylated hydroxy anisole (BHA).According to acquired results, L shows effective DPPH
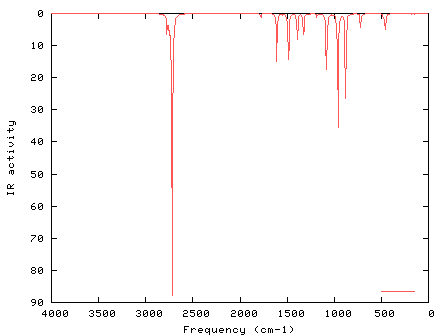
It worthy of note that, the radical scavenging activities of L were examined by using ABTS Frontier molecular orbitals, electronic absorption wavelengths and non-linear optical features of the L were investigated with molecular modeling methods. X-ray diffraction and Hirshfeld surface analysis were done to examine the contribution of intermolecular contacts in crystal packing of L. X-ray diffraction investigation shows that the L crystallized in phenol-imine form with O H⋯N intramolecular hydrogen bond. The obtained calculation geometry was found to be in well consistent with the experimental geometry. Theoretical calculations of L were performed by using density functional theory (DFT) calculations with the B3LYP/6-311++G (d,p) level in the ground state. A synthesized Schiff base, (E)-4-nitro-2-phenol ( L), was prepared and characterized by FT-IR, single crystal X-Ray diffraction, NMR chemical shift and UV–Vis.


 0 kommentar(er)
0 kommentar(er)
